Clinical Trial Protocol


https://clinicaltrials.gov/ProvidedDocs/85/NCT01619085/Prot_000.pdf

https://clinicaltrials.gov/ProvidedDocs/52/NCT03235752/Prot_000.pdf
Example #2 provided as a guide, customize as needed: Flow diagram
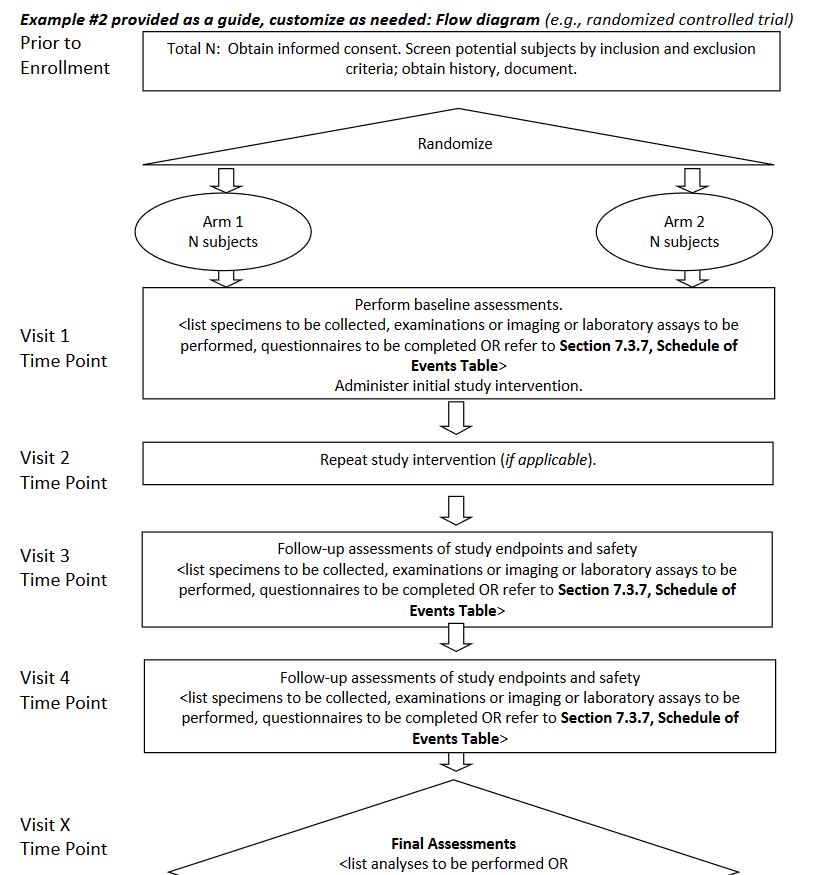
https://osp.od.nih.gov/wp-content/uploads/2014/01/Protocol_Template_05Feb2016_508.pdf
Clinical Study Protocol
Variability of Parkinson’s Disease Biomarker Analytes
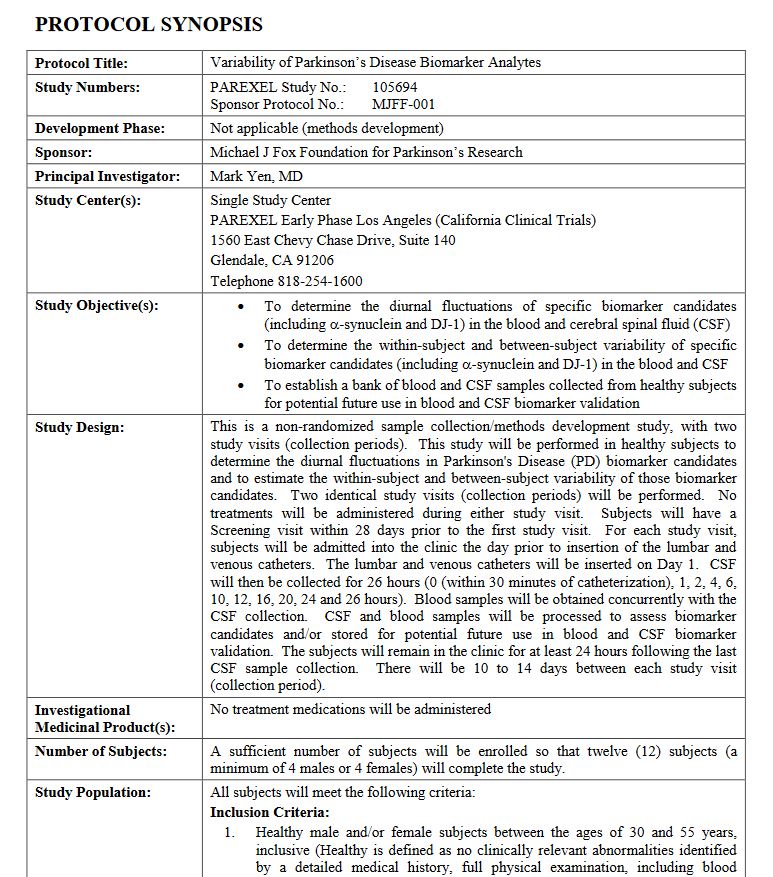
http://www.ppmi-info.org/wp-content/uploads/2011/09/Verification-study-in-control-subjects-protocol.pdf
Clinical Trials Protocol Templates
Applicants conducting phase 2 or 3 clinical trials that require Investigational New Drug applications (IND) or Investigational Device Exemption (IDE) applications can use a NIH-FDA template with instructional and sample text to help write protocols. A separate template is available for applicants conducting behavioral and social sciences clinical trials. Use of these templates is optional.
Purpose
To facilitate the development of clinical trial protocols that require a Food and Drug Administration (FDA) Investigational New Drug (IND) or Investigational Device Exemption (IDE) Application, the NIH and FDA collaboratively developed a Phase 2/3 Clinical Trial Protocol Template and an electronic protocol-writing tool to help investigators think through the scientific basis of their assumptions, minimize uncertainty in the interpretation of outcomes, and prevent loss of data.
Policy Guidelines &
Implementation
The tool provides a suggested format for phase 2 and 3 clinical trials funded by NIH that are being conducted under an FDA IND or IDE Application.
A common protocol structure and organization facilitates protocol review by oversight entities.
Note that the use of the tool is voluntary and is not required for NIH applications or contract proposals.
Notices
NIH and FDA Release Protocol Template for Phase 2 and 3 IND/IDE Clinical Trials
NIH Releases Protocol Template for Behavioral and Social Sciences Research Involving Humans
https://grants.nih.gov/policy/clinical-trials/protocol-template.htm





![[무료 자료]의학 용어 50선 & 의학 번역 참고 웹사이트 모음](https://static.wixstatic.com/media/9b595f_dc59ff36d7b54d518950b419983c0346~mv2.png/v1/fill/w_74,h_55,fp_0.50_0.50,q_95,enc_auto/9b595f_dc59ff36d7b54d518950b419983c0346~mv2.png)

![[의학] 의료 분야의 인공지능(AI)과 머신러닝(ML) 적용 사례 분석](https://static.wixstatic.com/media/9b595f_5349e526446146528dfb8d80c58617c1~mv2.jpg/v1/fill/w_74,h_49,fp_0.50_0.50,q_90,enc_auto/9b595f_5349e526446146528dfb8d80c58617c1~mv2.jpg)

![[의학] 로봇 지원 수술에 대한 최신 연구 동향 알려드려요.](https://static.wixstatic.com/media/9b595f_739e1ac71a6d4a078e1c7550d3cfd0d0~mv2.jpg/v1/fill/w_74,h_53,fp_0.50_0.50,q_90,enc_auto/9b595f_739e1ac71a6d4a078e1c7550d3cfd0d0~mv2.jpg)







